SATuRN and BioAfrica open access reports:
Here we provide you with sections from our open access annual reports, which are directed at public health practitioners, policies makers, doctors, researchers and the general public.
The objective of these reports is to present the key aspects of our work in an accessible, summarized format. The majority of the work presented has been published in peer-reviewed publications and can be seen in the publications section of krisp.org.za.
HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine
Authors: Hunt GM, Coovadia A, Abrams EJ, Sherman G, Meyers T, Morris L and Kuhn L.�
Objective:
To describe the prevalence of HIV-1 drug resistance mutations at the time of treatment initiation in a large cohort of HIV-infected children previously exposed to single dose nevirapine (sdNVP) for prevention of transmission.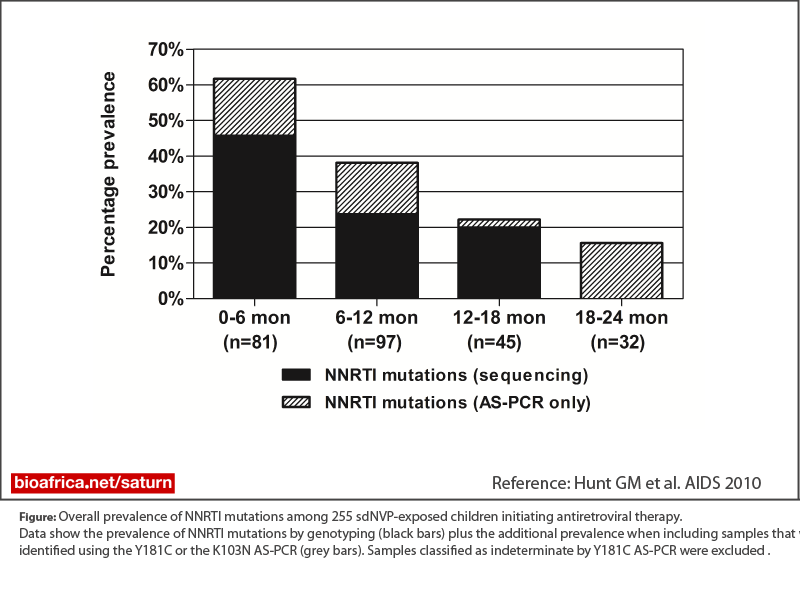
Design:
Drug resistance mutations were measured pre-treatment in 255 infants and young children under 2 years of age in South Africa exposed to sdNVP and initiating ritonavir-boosted lopinavir-based therapy. Those who achieved viral suppression were randomized to either continue the primary regimen or to switch to a nevirapine-based regimen. Pre-treatment samples were tested using population sequencing and real time allele-specific PCR (AS-PCR) to detect Y181C and K103N minority variants. Those with confirmed viremia >1000 copies/ml by 52 weeks post-randomization in the switch group were defined as having viral failure.�
Results:
Non-nucleoside reverse transcriptase inhibitor (NNRTI) mutations, predominantly Y181C, were detected by either method in 62% of infants less than 6 months of age, in 39% of children 6-12 months of age, 22% 12-18 months, and 16% 18-24 months (p=<0.0001). NNRTI mutations detected by genotyping, but not K103N or Y181C mutations detected only by AS-PCR, were associated with viral failure in the switch group.Conclusions:
The prevalence of mutations known to compromise primary NNRTI-based therapy is high in sdNVP-exposed children, supporting current guidelines recommending use of PI-based regimens for young children. Standard genotyping is adequate to identify children who could benefit from switching to NNRTI-based therapyLatest Reports
KRISP has been created by the coordinated effort of the University of KwaZulu-Natal (UKZN), the Technology Innovation Agency (TIA) and the South African Medical Research Countil (SAMRC).
Location: K-RITH Tower Building
Nelson R Mandela School of Medicine, UKZN
719 Umbilo Road, Durban, South Africa.
Director: Prof. Tulio de Oliveira




